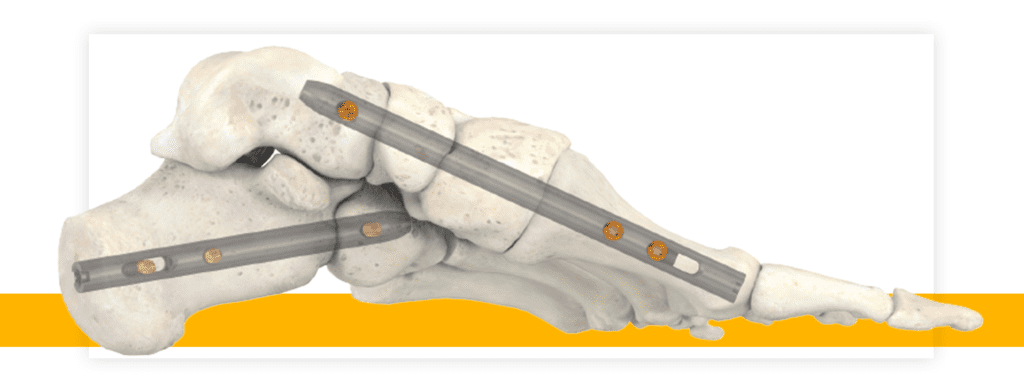
Accufix Surgical TM today announced that it has received approval from the U.S. Food and Drug Administration (FDA) to distribute its patented Accu-Joint system, a revolutionary new approach intended to enable full restoration of MTP function and motion, with no resecting or weakening of hard bone. The unique, non-weight bearing implant, allows surgical podiatrists to choose joint preservation over joint fusion for their patients. The Accu-Joint is intended to functionally and accurately restore arthritic toe joints, while allowing original anatomic bone structure to remain intact. It is also intended to be implanted with Hallux Valgus correction, providing that procedure is being performed.
Breakthrough Benefits
The Accu-Joint system consists of two patented technologies that separate it from competitors.
A unique two-stage MTP joint reamer removes worn cartilage, smooths bone spurs and countersinks the hard subchondral bone end of the toe joint, resulting in complete resurfacing for precise implant seating and bone ingrowth, preserving bone anatomy and bone length. At the same time, second-stage blades produce 360-degree clearance around the implant articular edge, allowing the implant to articulate in a smooth manner.
Secondly, the implants include cancellous threaded, solid stems – not cannulated – intended to provide rigid fixation and help prevent influx of infection into the medullary canal, along with grooves and scalloped edges under the articular head of the implant to allow bone ingrowth for reliable anti-rotation. The threaded “barrel” of the stem, just beneath the articular surface, transfers all stress forces off the stem, and away from the softer medullary canal, into the hard subchondral bone where it is rigidly fixated. The implants are sized smaller than the diameter of the bone end, which together with 4-points of internal rigid fixation, is intended to ensure that the bone – not the implant – bears the full weight of the body, while delivering the strength and implant stability necessary for full foot function.
True Anatomical Joint Preservation
“As a practicing podiatrist for 36 years, I have been dissatisfied with joint replacement implant options for my patients.” recalls Dr. Glenn C. Vitale, the inventor of the Accu-Joint. “I have surgically removed many failed implants and have chosen not to use any of the joint implants that are currently on the market today.”
“We finally have the opportunity to choose true anatomical joint preservation over joint fusion.” Fusions represent approximately 80% of current MTP procedures, yet are prone to reduced range of motion and can inhibit quality of life and limit the choice of shoe gear.